23 Apr Fentanyl patches recalled because of product mislabeling
Alvogen Inc. is voluntarily recalling two lots of Fentanyl Transdermal System opioid patches.
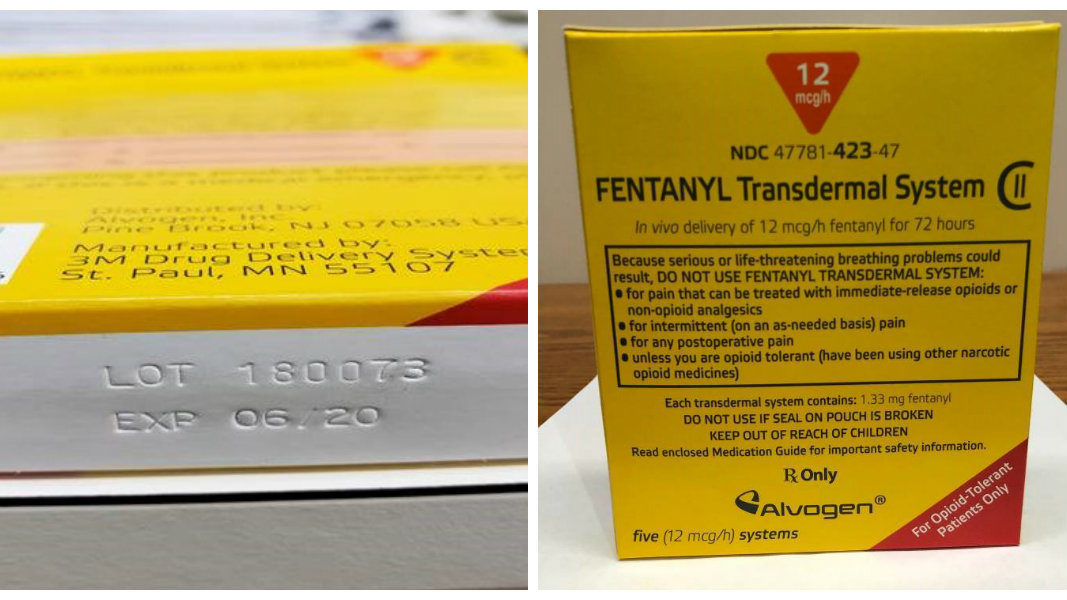
A small number of cartons labeled “12 mcg/h Fentanyl Transdermal System patches” actually contained 50 mcg/h patches, so Alvogen is recalling the 12 mcg/h lots.
The 50 mcg/h patches that were included in cartons labeled “12 mcg/h” are individually labeled as 50 mcg/h. This transdermal system is manufactured by 3M Drug Delivery Systems in St. Paul, Minnesota.
View more information from Business Wire.
Application of a 50 mcg/h patch instead of a prescribed 12 mcg/h patch could result in serious, life-threatening or fatal respiratory depression.
Groups that could face increased risk include first-time recipients of such patches, children and senior citizens.
Alvogen has not received any reports of problems related to this issue.
The product is meant for the management of pain in opioid-tolerant patients and is packaged in cartons of five individually wrapped and labeled pouches.
The affected Fentanyl Transdermal System lots include:
- Lot 180060 of Fentanyl Transdermal System, 12 mcg/h, expiration date 05/2020.
- Lot 180073 of Fentanyl Transdermal System, 12 mcg/h, expiration date 06/2020.
The mislabeled product is packaged in a 12 mcg/h primary carton. These lots of Fentanyl Transdermal System were distributed to pharmacies across the country.
Alvogen is notifying its distributors and direct customers by certified letter and is arranging for return and replacement of all recalled products. Pharmacies are requested not to dispense any product subject to this recall. Patients who have this recalled product should immediately remove any patch currently in use and contact their health care provider. Patients with unused patches should return them to the place where they were purchased, for replacement.
Questions should be directed to Alvogen Customer Complaints by calling 866-770-3024 or sending an e-mail to the company.
The company is available from 9 a.m. to 5 p.m. Eastern Standard Time, Monday to Friday.
People are advised to contact their physician or health care provider if they have experienced any problems that may be related to taking or using this drug.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
If you’re going to mail or fax it, download the form here, or call 800-332-1088 to request one. Complete and return it to the address on the pre-addressed form, or submit it by fax to 800-FDA-0178.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Copyright 2019 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed.
[ad_2]
Source link



No Comments